The bioequivalence study involves administration of the drug product under investigation and the to healthy normal volunteers in a design.
In this case, each subject acts as his/her own control. Time should be allowed between the administration of each product, to make sure that all the drug from the previous administration has been completely eliminated (washout period).
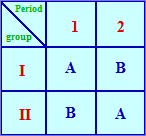
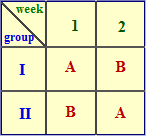
Balanced Crossover Design
Subjects involved in the study are divided into two groups I and II. Group I receives product A and group II receives product B. After the washout period, group I should receive product B, and group II should receive product A. This way individual variation and variation due to sequence of drug administration are eliminated.